Sterile and Compliant Lens - Canada
Sterile and compliant lens for Eagle V1.2 Imaging System™ with LEXAN™ 8040 Film

.png)
Sterile and compliant lens for Eagle V1.2 Imaging System™ with LEXAN™ 8040 Film
Approximately 1 in 5 women still require a second surgery, following their index breast conserving surgery, in order to achieve clean surgical margins.
The Eagle V1.2 Imaging System™ is an investigational intra-operative fluorescence imaging device, which will be used to visualize the distinct red fluorescence produced by cancer cells, against the natural green fluorescence of healthy breast tissues.
Our FDA approved LEXAN™ 8040 film is used as a disposable lens cover placed on the system to overcome the high refractive index of glass which was causing distortions in the images seen in real time.
Extensive testing was executed on different kind of plastics, on:

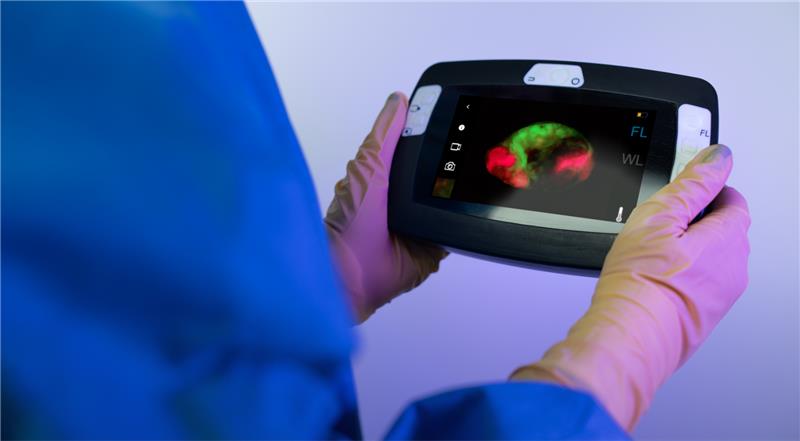
* The Eagle V1.2 Imaging System™ is still in phase III clinical trial
| TITULO | Tipo de recurso | Idioma | Região |
|---|
Client:
It is their mission to address this requirement in care - by providing breast cancer surgeons with the ability to visualize residual disease in real-time during index breast cancer surgeries. Driven by this mission, SBI ALApharma Canada Inc. is conducting a pivotal Phase III clinical trial to assess the use of fluorescence-guided surgery for breast cancer patients.
Partner:



Para encontrar um distribuidor em sua área, insira o seu país.
ENCONTRE SEU DISTRIBUIDORPara encontrar um distribuidor em sua área, por favor, selecione o país
Encontre um distribuidor